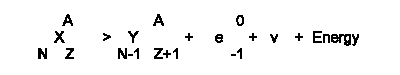Home|
Other
Links|
School Info|
Image
Gallery|
Questions
Answers to Sample Certification Questions
I. Radiopharmacy and Nuclear Medicine
Physics
II. Clinical Procedures
Radionuclide Generators
-
Correct answer: a. Na pertechnetate is the most
highly oxidized chemical form of Tc, in which the oxidation number is 7+.
Choice b is incorrect. Hydrolyzed reduced Tc is an insoluble colloid
localizing in the RES. Choice c is also incorrect and represents
an insoluble form. Choice d is impossible since sulfate is not used
to elute the generator.
-
Correct answer: c. Transient equilibrium is reached
when the parent's half-life is approximately 10 times that of the daughter.
In this case, the half-life of Mo-99 (67 hr) is 11 times that of Tc-99m
(6 hr), producing a ratio of 11:1. The Mo/Tc generator is considered an
ideal example of transient equilibrium.
Choices a and d are incorrect since
they don't even represent types of equilibrium. Choice b is also
incorrect since the requirement for secular equilibrium is that the half-life
of the parent be >> half-life of the daughter, e.g., 100:1 or greater.
-
Correct answer: d. The daughter can't decay until
it is formed. Since the rate of formation of the daughter is slow, in the
equilibrium mixture, the daughter will appear to decay with the
parent's half-life. When separated from the mixture (e.g., when the generator
is eluted), the daughter decays with its characteristic half-life.
-
Correct answer: c. Soluble "aluminum ion breakthrough"
can, under certain conditions, appear in the eluate. While not harmful
to the patient, it interferes with preparation of Tc-bone agents, Tc-sulfur
colloid, and Tc-RBC labeling. It is therefore mandatory to prove that the
level of this chemical impurity does not exceed 10 ppm. It is also possible
that the eluate will contain a small amount of Mo-99. This is called "Mo-breakthrough"
and must not exceed 0.15 mCi Mo-99/mCi Tc-99m at time of administration.
Testing for this radionuclide impurity is mandatory for all licensees.
It is also possible that colloidal "hydrolyzed reduce Tc", a radiochemical
impurity that is taken up in the RES, will be present in the eluate. While
not mandatory to quantify its presence, this simple, rapid, inexpensive
test is highly recommended.
-
Correct answer: d. The actual time required to
reach maximum activity level in the Mo/Tc generator is 23 hr. This is an
ideal equilibrium time since the generator is typically eluted every 24
hr, right after equilibrium is reached. This maximizes the yield of pertechnetate
removed from the generator.
Back to Questions
|
List of Contents
Physics of Nuclear Medicine
-
Correct answer is "d". All other answers are
incorrect since the other "iso" terms require one number to be constant
and the other two numbers to be variable. Isomers can never be isotopes,
isotones, or isobars.
-
Correct answer is "a". In b- decay, an electron
is emitted from the nucleus. Other than the source (nucleus rather than
outer orbitals), it is indistinguishable from an ordinary electron.
-
Correct answer is "a". Gamma rays and X-rays
of the same energy have almost identical interactions in matter. The primary
difference is their source: gammas are emitted from the nucleus; X-rays,
from the outer orbitals.
-
Correct answer is "a". In beta-minus decay, the
Z number of the daughter always increases by one unit to counterbalance
the -1 charge on the emitted electron. There is only negligible change
in mass. The generic equation representing this decay is shown below:
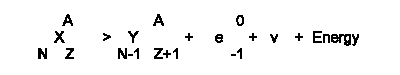
-
Correct answer is "d". 1 mCi = 10-3
Ci X 3.7 X 1010 dps/Ci = 3.7 X 107 dps
Back to Questions
|
List of Contents
Math Problems
-
Correct answer is "b". Logically, 100 mCi to 50 mCi
to 25 mCi requires two half-lives. If 24 hr = 2 half-lives, then the half-life
= 12 hr. Mathematically,
At = A0 X 0.5 (elapsed
time/half-life)
therefore
25 = 100 X 0.5 (24 hr/t) and 0.25
= 0.5 (24/t).
Taking the log of both sides,
log 0.25 = (24 X log 0.5)/t
therefore
t = (24 x log 0.5)/log 0.25
so
half-life = 12 hr
-
Correct answer is "b". Any time one half-value
layer is removed from between the source and the detector, the intensity
reading doubles. Since I0 is 10 mR/hr, new reading will be
20 mR/hr.
-
Correct answer is "b". Fraction remaining = 62.5/1000
= 0.0625 = 0.5 n where n = # of half-lives. Taking the log of
both sides, log 0.0625 = n x log 0.5 and n = log 0.0625/log 0.5 therefore
n = 4
-
Correct answer is "d". Fraction remaining = 0.5
# of half-lives = 0.519/6
-
Correct answer is "c". 40 hr/week x 50 weeks/yr
= 2000 hr/yr. Dose rate received is therefore 5000 mR/2000 hr = 2.5 mR/hr.
By inverse square law, 40 mR/hr x (1 ft)2 = 2.5 mR/hr x d2.
d2 = 16 ft2 and therefore d = 4 ft
Back to Questions
|
List of Contents
Radiopharmacy
-
Correct answer is "b". This mechanism is also
referred to as capillary blockage.
-
Correct answer is "b". Particulate blockade refers
to capillary blockade; hydrolysis of colloid particles has nothing to do
with liver/spleen uptake and may not even take place.
-
Correct answers are "a, b, d". I125
can not be imaged due to the low energy of its gamma and X-rays (28 and
35 keV)
-
Correct answer is "d". Tl1+ is a K1+ analog;
its distribution is directly proportional to blood flow. Choice "b" is
incorrect- Tl-201 is excluded from acutely infarcted myocardium.
-
Correct answer is "a". It is the only reducing
agent in the list.
Back to Questions
|
List of Contents
Quality Control
-
Correct answer is "b". System "a" is reversed.
Systems listed in "c" and "d" are suitable for hepatobiliary agents, but
not for the simpler radiopharmaceuticals, e.g., MDP, DTPA, SC, GH, MIAA,
pertechnetate.
-
Correct answer is "d". Hydrolyzed reduced Tc,
Tc-MAA, and all other insoluble compounds tend to stay where they are spotted
on the chromatography strip. Since they don't migrate, they are inseparable
and therefore this impurity is not quantifiable in the presence of insoluble
compounds.
-
Correct answer is "b". Determination of unshielded
Tc-99m activity first leaves a charge on the ionization chamber that takes
up to 4-5 minutes to bleed off. Artifactually high readings of Mo-99 are
likely to be obtained even if no Mo-99 is present, causing a test failure.
-
Correct answer is "a". Thyroid monitoring is
required at 24 hours. None of the other choices represents a legal requirement.
Back to Questions
|
List of Contents

back to homepage